Second COVID-19 vaccine approved for use in the UK
More people are eligible to receive the COVID-19 vaccine
This week, a second COVID-19 vaccine was approved for use in the UK and the guidance about how to use the first vaccine was updated.
Because of this, we can expect the supply of vaccines available in the UK to increase substantially in the coming weeks. This in turn will allow a higher rate of vaccine deployment to all parts of the country, to allow more people to receive the vaccine as soon as possible.
Second vaccine approved
Oxford/AstraZeneca vaccine
The body that regulates and approves medicines and vaccines in the UK, the MHRA, approved the first vaccine to help protect us from COVID-19 on 02 December 2020. On 30 December, it announced that a second vaccine, the Oxford/AstraZeneca vaccine, was approved after meeting the required safety, quality and effectiveness standards.
A key benefit of this new vaccine is that it can be stored in regular refrigerated conditions, making it easier to deploy to existing healthcare settings, whereas the Pfizer/BioNTech vaccine needs to be stored at a very low temperature (-70°C).
Changes to the use of the Pfizer/BioNTech vaccine
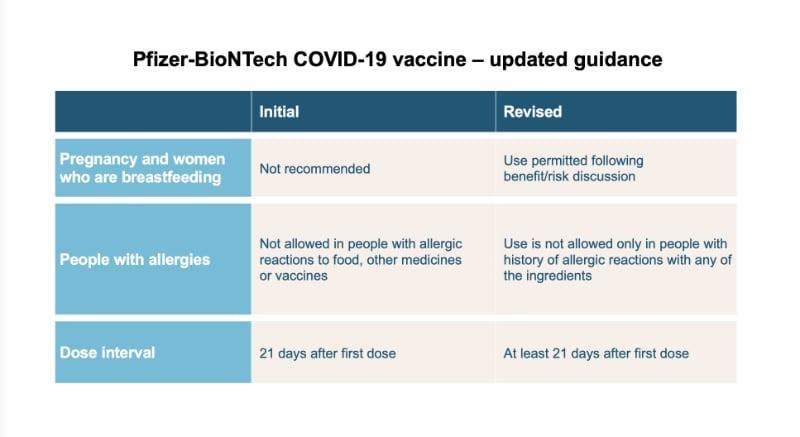
The Commission on Human Medicines (CHM) has reviewed further data for the Pfizer/BioNTech vaccine as it has become available and has recommended the following changes:
Pregnancy and women who are breastfeeding
This Pfizer/BioNTech vaccine can now be considered for pregnant and breastfeeding women based on the individual benefits and risks for each person - to be discussed with your doctor (previously not recommended at all in pregnancy or breastfeeding).
Allergies
Anyone with a previous history of allergic reactions to the ingredients of the Pfizer/BioNTech vaccine should not receive it, but those with any other allergies such as a food allergy can now have the vaccine (previously not recommended for anyone with a history of allergic reactions to food, other medicines or vaccines).
Dosage interval
The dose interval for the Pfizer/BioNTech vaccine is now at least 21 days after the first dose (previously 21 days)
What does this mean for PSC patients?
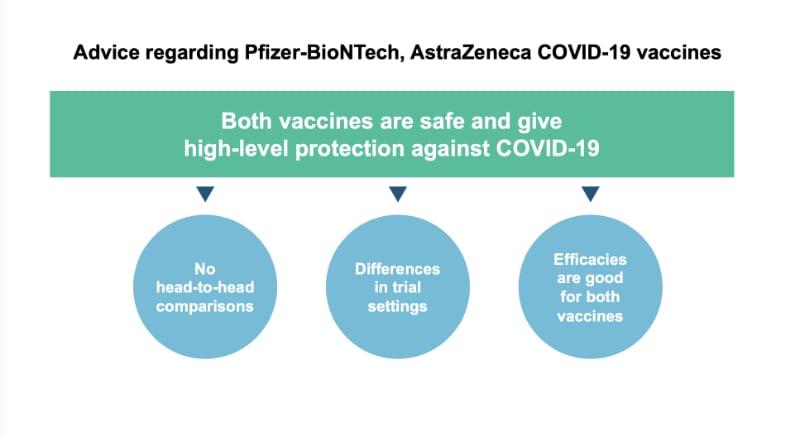
Two vaccines are now available to help protect us from COVID-19. The overall advice from JCVI (independent scientific group that advises on vaccine use) is that both vaccines are safe and give high-level protection against COVID-19 in adults.
It is not possible to say whether one is better than the other because the clinical trials were set up in different ways, meaning they cannot be compared on a like-for-like basis. The JCVI does not advise a preference for either vaccine in any specific population.
Priority Groups
The COVID-19 vaccination programme is already being rolled out across each of the four nations, and certain groups have been prioritised to receive the vaccine first. People are currently prioritised according to age, health status and some occupations but this may change as new data is reviewed. Some people with PSC are currently in the priority groups.
First Dose for More People
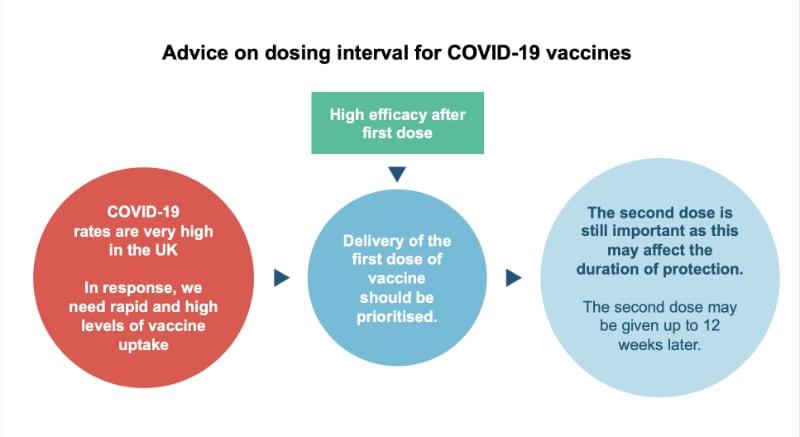
Because COVID-19 rates are very high in the UK, rapid and high levels of vaccine uptake are needed. The JCVI therefore recommends that delivery of the first dose of vaccines should be prioritised. This will allow the greatest number of people who are eligible to receive the vaccine to get it in the shortest time possible (as opposed to providing the required two doses to fewer people in the same time frame). This strategy is expected to protect the greatest number of lives.
Second Dose
Everyone will still receive their second dose and this will be within 12 weeks of their first. The second dose completes the course and is important for longer term protection.
Faster Rollout
Because of the new Oxford/AstraZeneca vaccine, the recommended changes in the use of the Pfizer/BioNTech vaccine, and the prioritisation of the first dose of vaccines to those in the most high-risk groups, more people can be vaccinated, quicker. You may be called very soon for your vaccination.
Our ‘Vaccines for COVID-19’ page is a quick and easy read on COVID-19 vaccines and who is in which priority group. We also include links to our recent live Q&A on vaccines and statements about vaccines from various professional societies.
News About COVID-19 Vaccines
COVID Vaccine and Transplantation
The British Transplantation Society has issued a Position Statement confirming that it supports vaccination for COVID-19 for transplant patients.
COVID Vaccine and Autoimmune Liver Disease
Video of our live Q&A with Dr Trivedi on the COVID vaccine and autoimmune liver disease.
Priority Groups for COVID-19 Vaccines
**UPDATED 02 DEC** Who will be prioritised to receive the COVID-19 vaccine?
Coronavirus Vaccine: Reasons to be Optimistic
Reasons to be optimistic as the world races to find a vaccine for the new coronavirus