Liver Perfusion Could Save 7 in 10 Rejected Donor Livers
Liver Perfusion Could Save 7 in 10 Rejected Donor Livers
New research from the University of Birmingham reveals results of clinical trial
Normothermic Machine Perfusion of the Liver (NMPL)
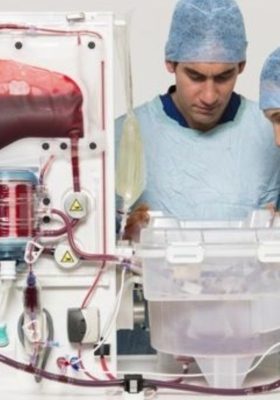
New Technology Improves Donor Livers
A research study called the VITTAL trial, conducted in the Queen Elizabeth Hospital, Birmingham, has shown that using a new technology called normothermic machine perfusion of the liver (NMPL) means that more donor livers can be used for transplantation. Potentially, nearly three quarters of livers that would not normally be good enough could be used to improve and save lives.
Dr Richard Laing, one of the study's authors, told PSC Support, "Machine perfusion has the potential to change the face of transplantation. Following on from the success of the VITTAL trial, surgeons are now using this technique to safely utilise a broader range of donor livers for patients who need them most."
What is Normothermic Machine Perfusion of the Liver?
Normothermic machine perfusion of the liver (NMPL) is a new technology used in liver transplantation that supplies the donor liver with oxygen and nutrients at body temperature. It helps prevent the cell damage seen when the liver is cooled to ice temperature (static cold storage) and allows doctors to study how well the donor liver is able to function. This means that doctors can identify livers that are suitable for transplant and discard fewer organs.
Why is the VITTAL Clinical Trial Important?
Liver transplantation is a life-saving treatment for some people with PSC. However, the current supply of donor livers doesn't meet demand and there is an urgent need to expand the pool of available livers. A third of donated livers don’t meet the necessary standards to be used for liver transplant and are therefore not used for transplants.
This decision not to use a donor liver is usually based on the history of the donor and the professional views of the transplanting surgeon. Suitable livers are preserved using cold static preservation (keeping the liver very cold). Cold static preservation does not allow for any assessment of liver function, and the only other source of information about the donor liver is from tissue samples (which can help identify fatty livers which are not suitable). This means many livers are discarded at the last minute and cannot be used.
NMPL does allow the function of the liver to be assessed, and can actually improve how well that liver functions.
The VITTAL study showed that 7 out of 10 livers that would not normally be used were improved with NMPL, and used successfully in liver transplants. If the use of NMPL becomes everyday practice, then more livers will be available for transplants, and there could be shorter waiting times for transplants. With NMPL, the donor liver can be kept in good condition for much longer than using cold static preservation, allowing transplant centres to better plan and time transplantation operations, especially if other transplants are already going ahead at that transplant centre.
Game Changer
More livers, shorter waiting times and better timed operations is game-changing for people who are on the waiting list for a liver transplant. A number of transplant centres already have the NMPL technology in place, and with adequate intensive care resources, and presumed consent legislation, there could be more life-saving transplants conducted than ever before. This is good news for people with PSC facing transplantation.
VITTAL Clinical Trial
The research team evaluated donor livers that were not suitable for use in transplantation and matched them with patients on the liver transplant waiting list who had volunteered to take part. Transplant centres had already declined to use these livers for various reasons, including:
- the transplant team were already busy with other transplant operations
- lack of suitable recipients
- delays with transportation
- livers being too fatty
- livers being damaged
164 patients on the waiting list were approached to take part, of which 53 agreed, and 22 were enrolled in the study. The leading indication for transplantation in the volunteers was alcohol-related liver disease (36%), followed by primary sclerosing cholangitis (27%) and non-alcoholic steatohepatitis (NASH)(18%).
Out of 31 livers, 22 (71%) improved enough to be transplanted after NMPL. They were transplanted after a median (average) preservation time of 18 hours, with 100% 90-day survival.
Taking part was a big decision for everyone, at a time of great uncertainty, and without the bravery of each participant, we would not have the evidence and knowledge today about the benefits of NMPL. Thank you to everyone who took part in this and other clinical trials to develop new medicines and technologies.
