Last year, you responded to our PSC Support Patient Insights Survey to help understand PSC healthcare and to shape PSC research design. Thank you to everyone who took the time to give answers. We shared the responses to the individual questions with all stakeholders immediately after the survey closed, part 1 of our report earlier this year and now we're bringing you the second part of the survey report.
Part 1 is about PSC healthcare.
Part 2 is about attitudes to research and study design (below).
Patient Insights Report
Part 2 - PSC Clinical Trials
Background and Recommendations
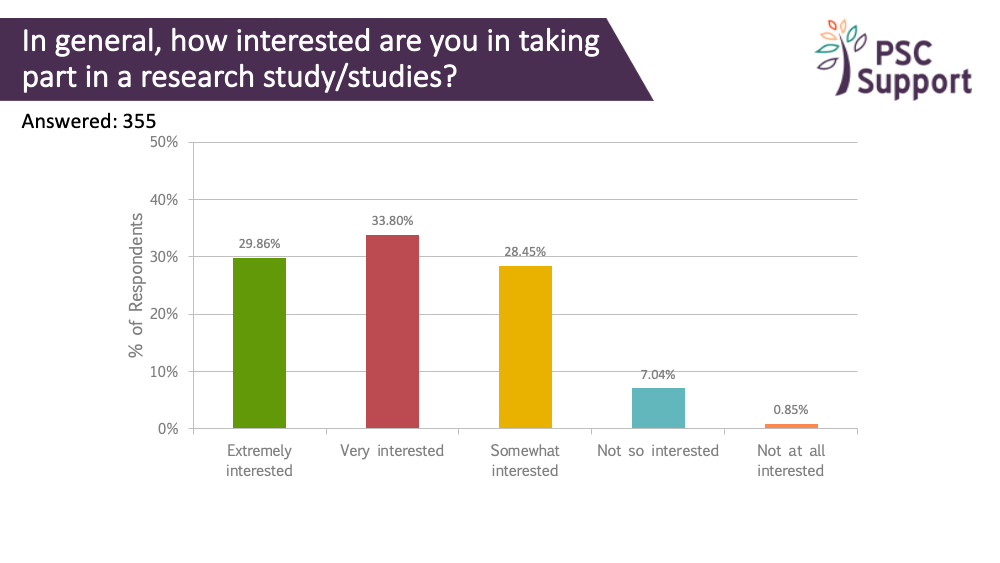
There is strong interest from PSC patients in taking part in research. The unmet needs of PSC patients and their urgent need for an effective treatment is well-established thanks to your responses in our past surveys, and PSC Support and other patient groups have communicated this to pharmaceutical companies, regulatory authorities, healthcare professionals and scientists. There is a great deal of interest right now in developing drugs for PSC, and we're working closely with researchers and pharmaceutical companies to help find an effective treatment for PSC. There are a number of clinical trials running and in the pipeline.
The risk of not recruiting
However, PSC is a rare disease and every clinical trial risks being unable to recruit the necessary numbers to demonstrate efficacy of the treatment drug, despite the positive attitudes from patients about taking part. If trials don't recruit enough patients, we will never get a cure.
We have seen a demand for clinical trials, but there are a number of barriers preventing or putting off otherwise willing and eligible participants from taking part.
Removing the barriers
However, these barriers can be addressed to make trials more accessible in the future. This PSC Support Patient Insights report identifies motivations for and barriers to taking part in research and makes recommendations about how researchers and pharmaceutical companies can change trial design to maximise recruitment.
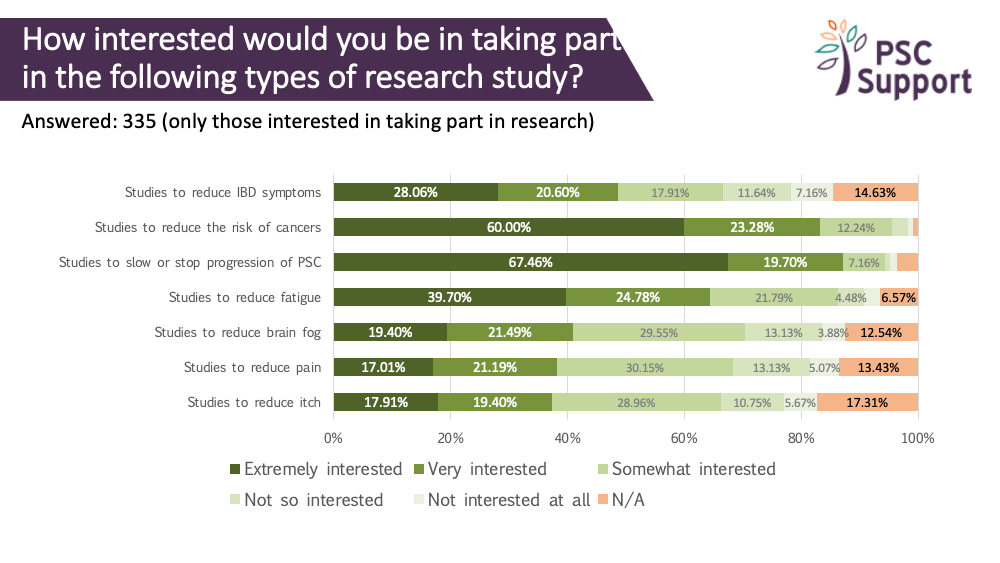
Of those interested in taking part in research, 87.16% were extremely interested (67.46%) or very interested (19.70%) in taking part in research to slow or stop progression of PSC.
Of those interested in taking part in research, 83.28% were extremely interested (60.0%) or very interested (23.28%) in taking part in research to slow or stop progression of PSC.
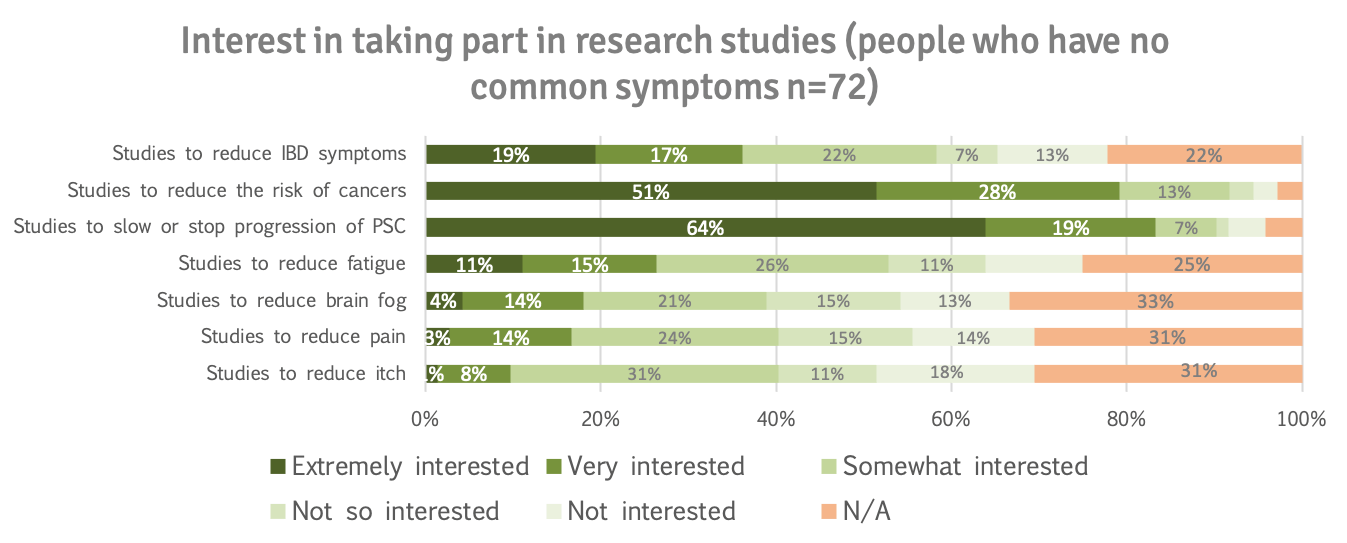
Patients who are experiencing symptoms are especially interested in taking part in symptom research.
Even patients who don't have symptoms want to take part to slow or stop progression of the disease (83%) or lower the risk of cancer (79%).
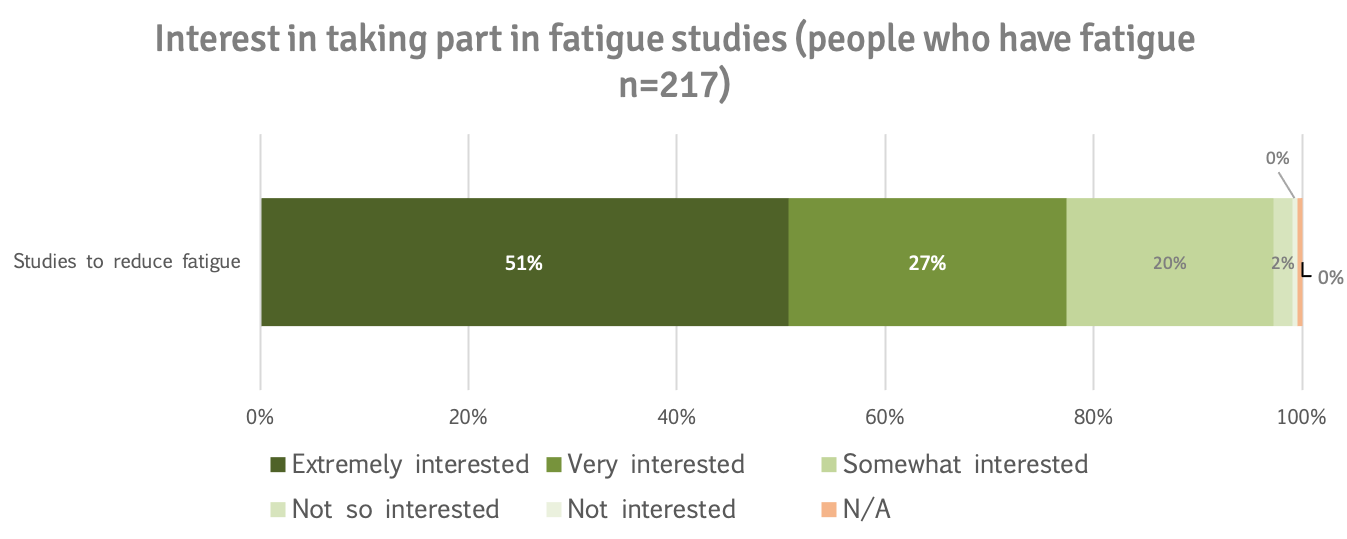
78% of patients who indicated they had fatigue were extremely (51%) or very interested (27%) in taking part in studies to reduce fatigue.
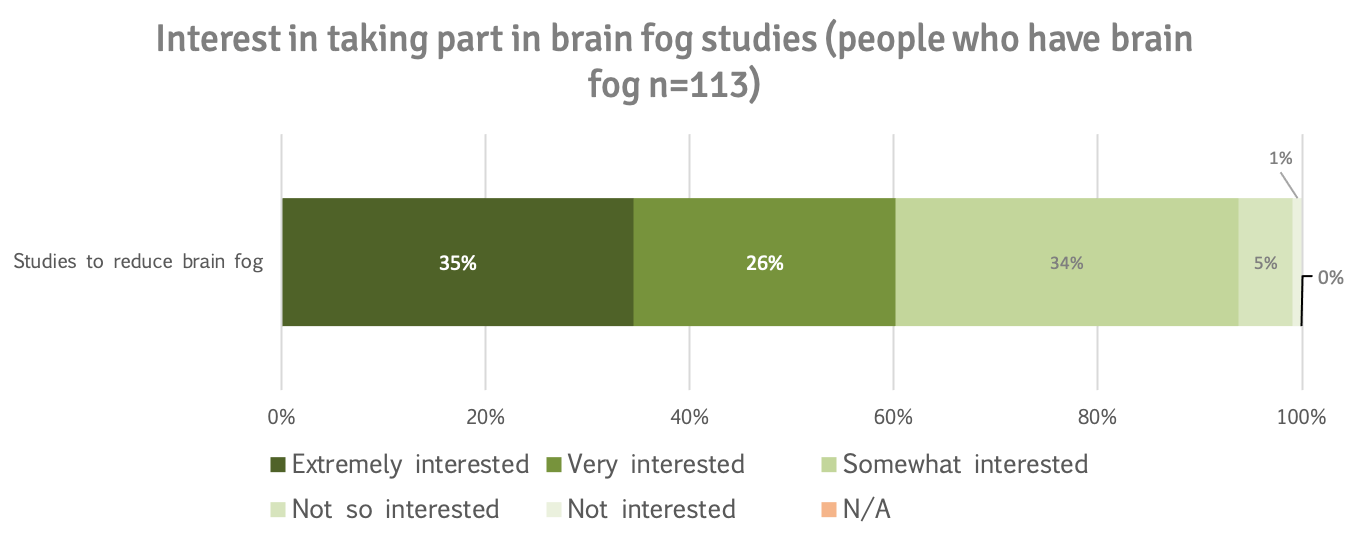
61% of patients who indicated they had brain fog were extremely (35%) or very interested (26%) in taking part in studies to reduce brain fog.
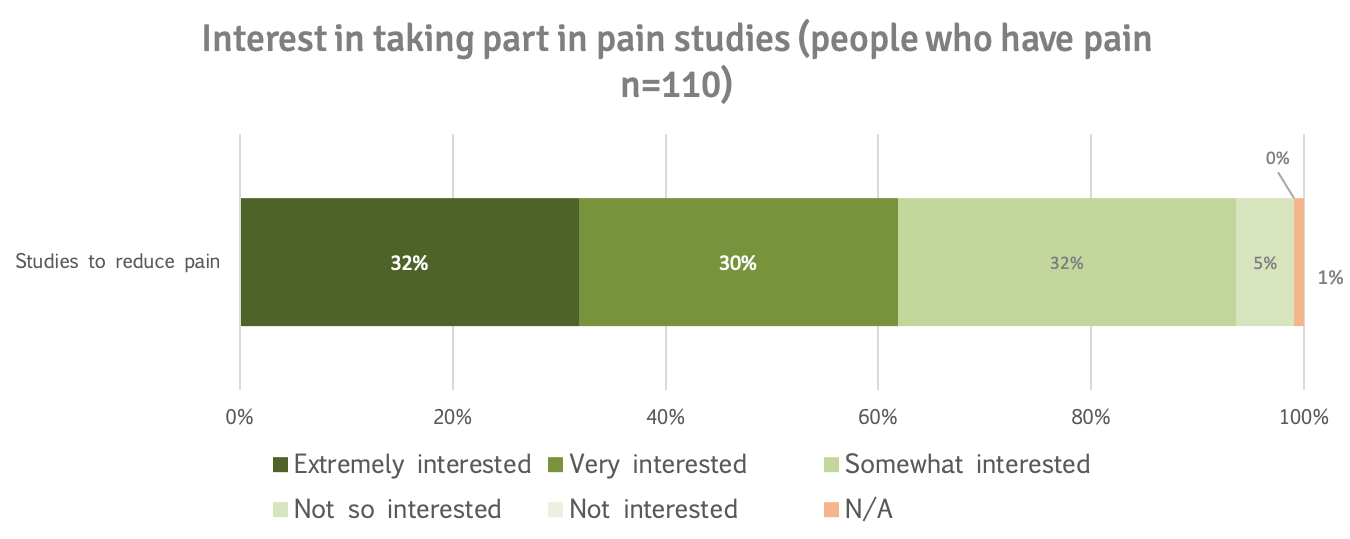
62% of patients who indicated they had pain were extremely (32%) or very interested (30%) in taking part in studies to reduce pain.
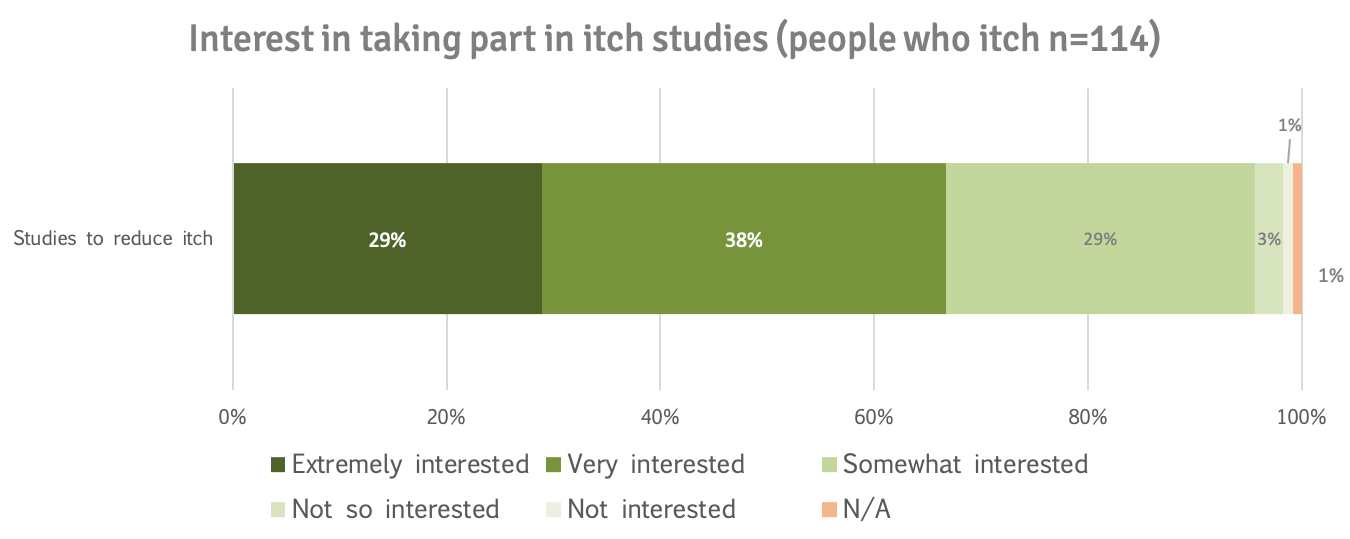
67% of patients who indicated they had itch were extremely (29%) or very interested (38%) in taking part in studies to reduce itch.
Motivation for taking part in clinical trials
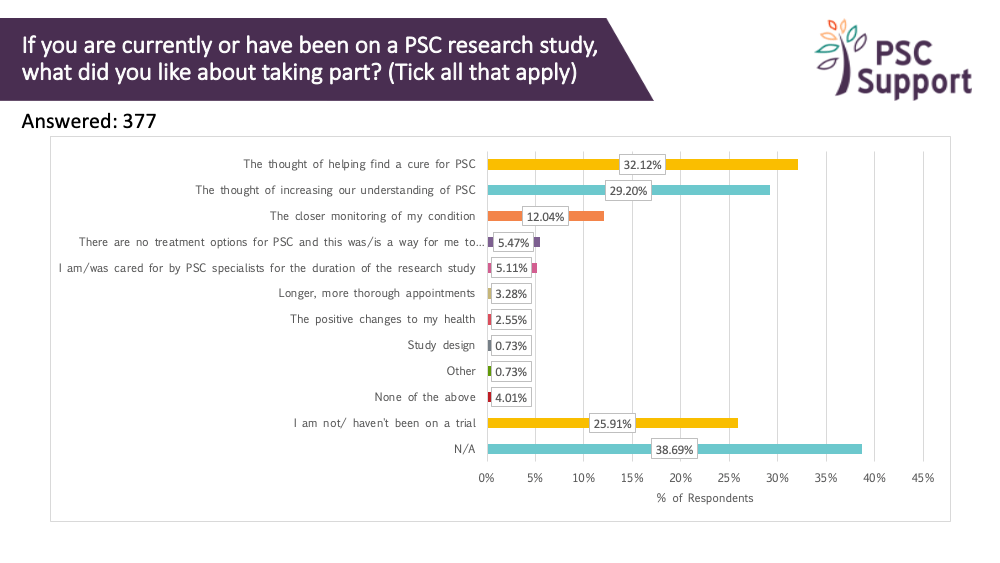
We asked everyone what they liked about taking part in trials, if they'd been on one. 39% reported 'not applicable' or that they hadn't been on a trial (26%). The three most frequently cited reasons were:
- the thought of helping find a cure for PSC (32%)
- the thought of increasing our understanding of PSC (29%)
- the closer monitoring of the condition (12%)
Supporting insights
Learning about clinical trials
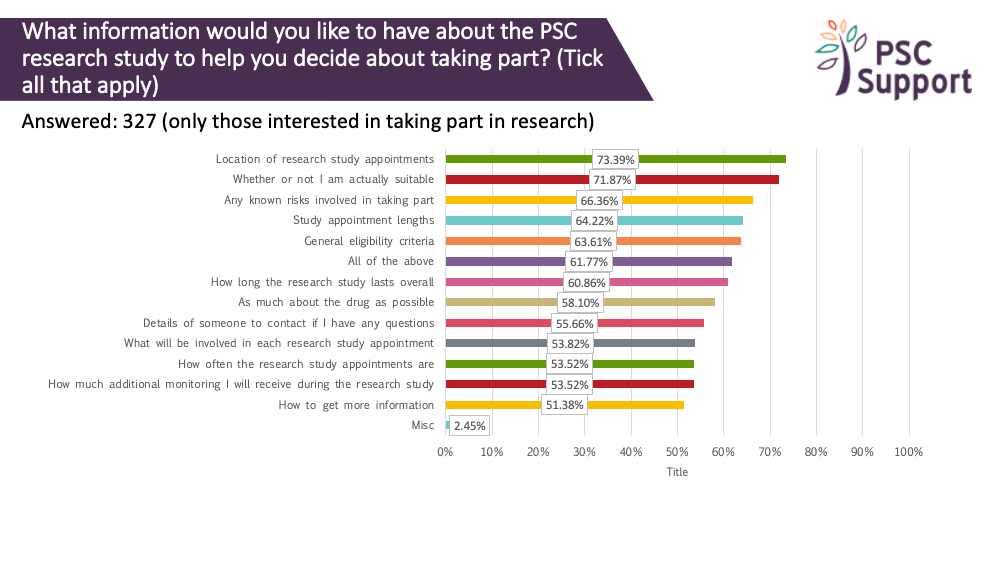
What patients want to know
We asked what information about a research study people would like to know before deciding whether to take part. The responses showed that people with PSC are keen to have as much information as possible before making their decision, especially about eligibility, risks of the drug and the logistics of taking part.
Recommendation 1
Potential participants should be given as much information as possible about a clinical trial before contacting the research team, including:
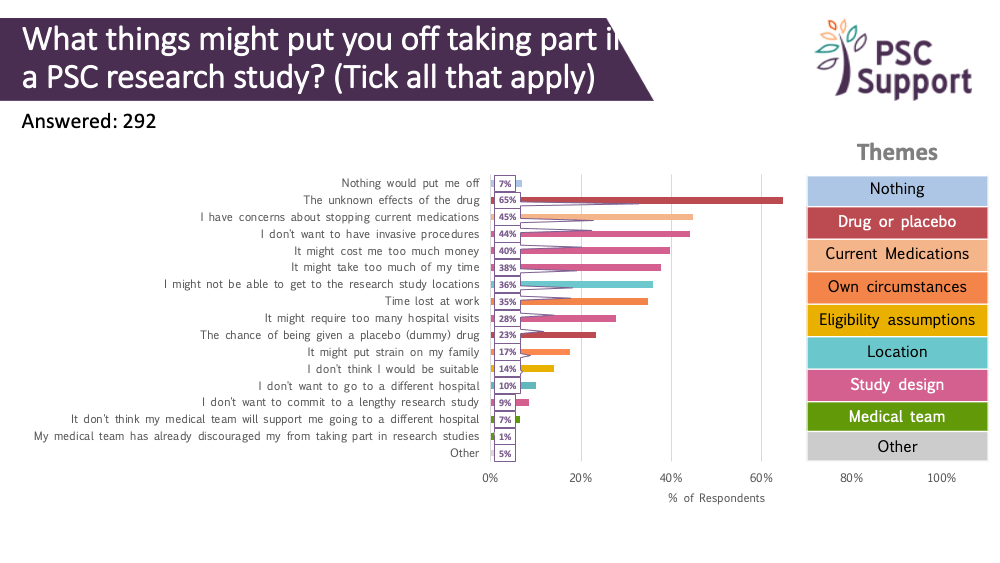
- information about the study drug and its mechanism of action, preferably explained by a trusted PSC healthcare professional in a video or written, easy to understand summaries of previously published and peer-reviewed findings. This includes studies of the same drug used in other populations. Going on a clinical trial is a big, risky decision for any patient. The biggest potential barrier is accepting the unknown risks of the treatment drug.
- key information about the trial itself, including the number of study visits, duration and what tests will be conducted. The population that meets eligibility criteria in PSC studies tends to be in their thirties and forties, and critically, working, often full time, and so managing the logistics of taking part is important. Taking days off work for study visits is a big ask.
Where patients find clinical trial information
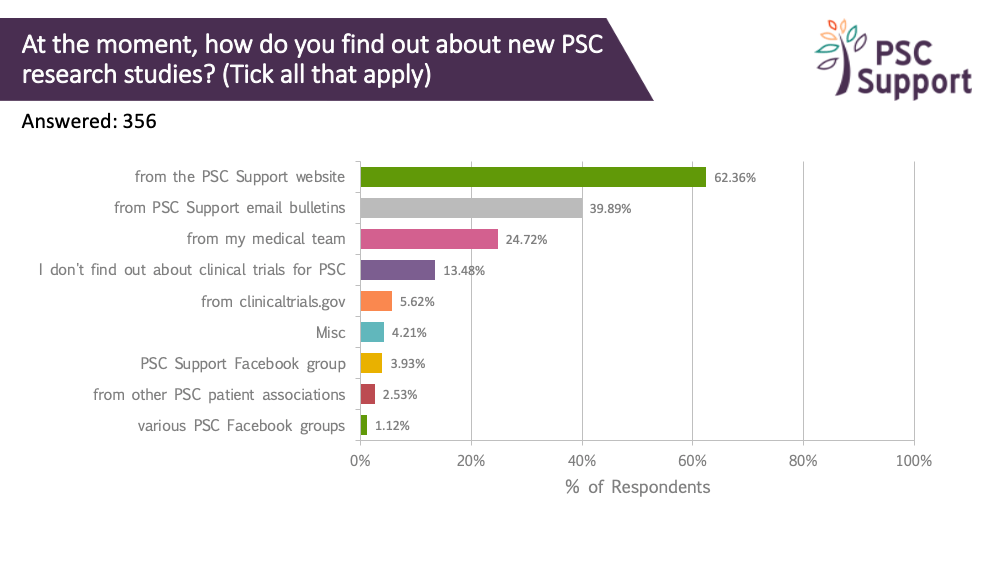
By far, most respondents get PSC research study information from PSC Support. We have a dedicated webpage for clinical trials and email bulletins specifically about research only. A quarter (25%) got this information from their medical team. Only 6% used clinicaltrials.gov.
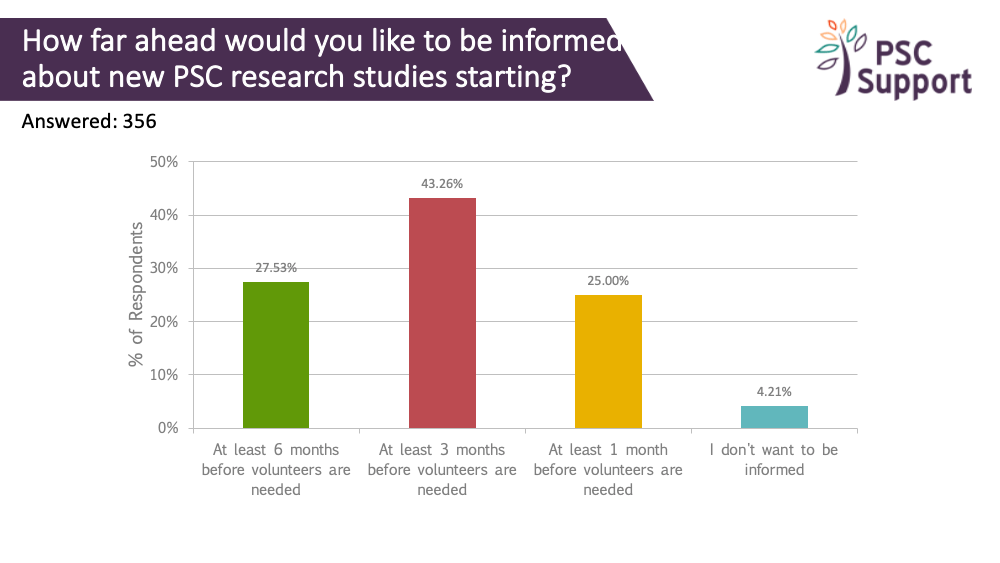
When patients want to find out about clinical trials
A quarter indicated that they would like to know about new PSC research studies at least 6 months before the study started recruiting, and 43% at least three months ahead. However, knowing about trials well in advance must be balanced against the risk of the clinical trial not going ahead. In 2019, a new clinical trial in the UK was stopped days before recruitment was due to start.
Recommendation 2
Investigators and sponsors should work with trusted patient groups like PSC Support to share information about new clinical trials at the earliest opportunity as long as there is strong confidence that the trial will go ahead, and individual study sites are properly signed up to run the study.
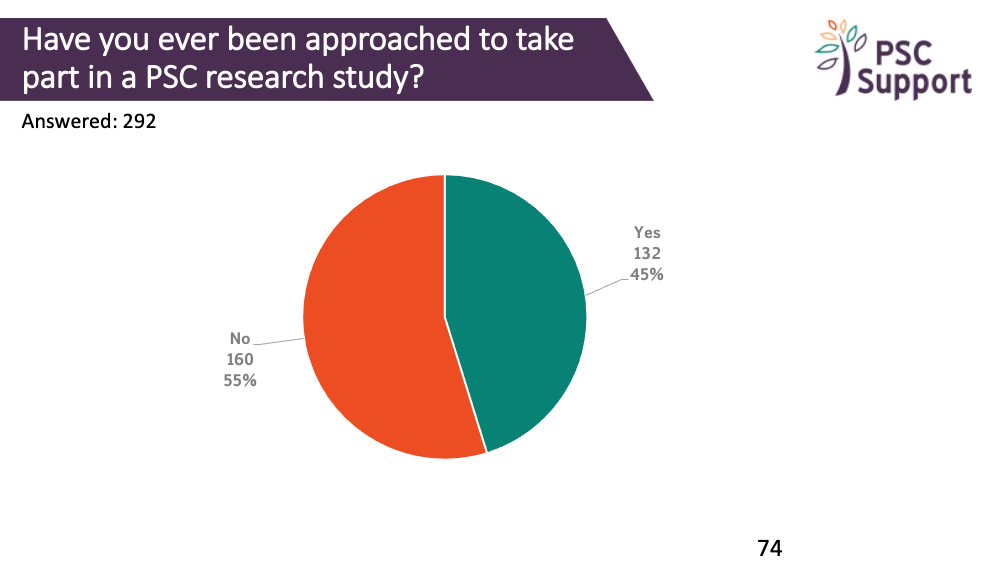
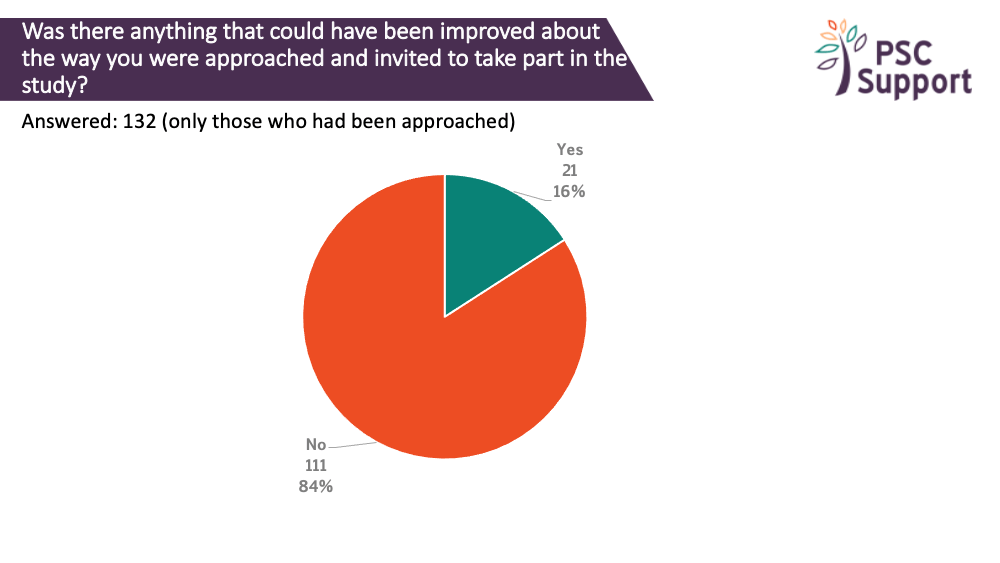
Inviting patients to take part in clinical trials
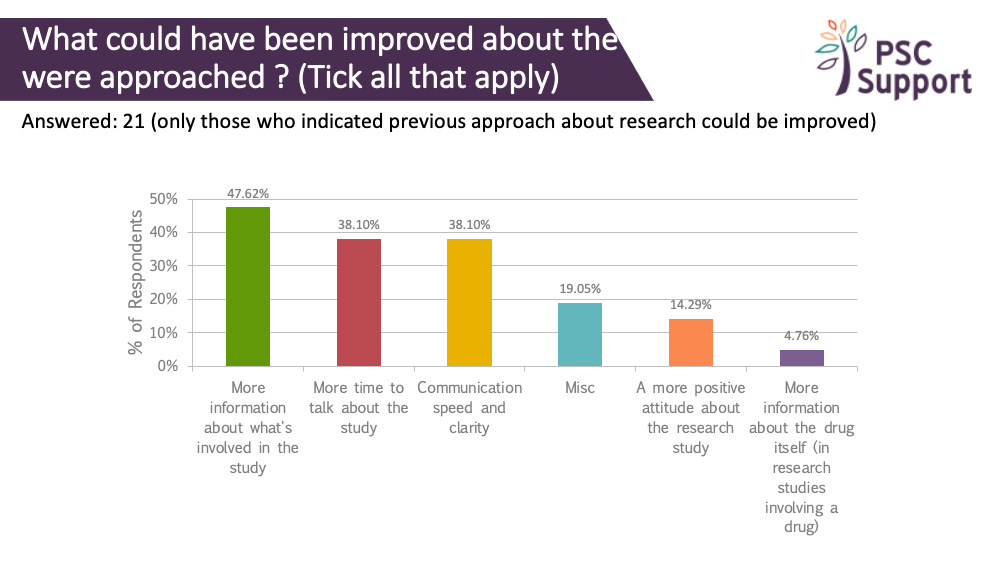
Of those who had been approached and invited to take part in a research study, some said this approach could have been improved. The main areas for improvement were around communication: having more information, more time to talk about the study and the speed and clarity of the communication.
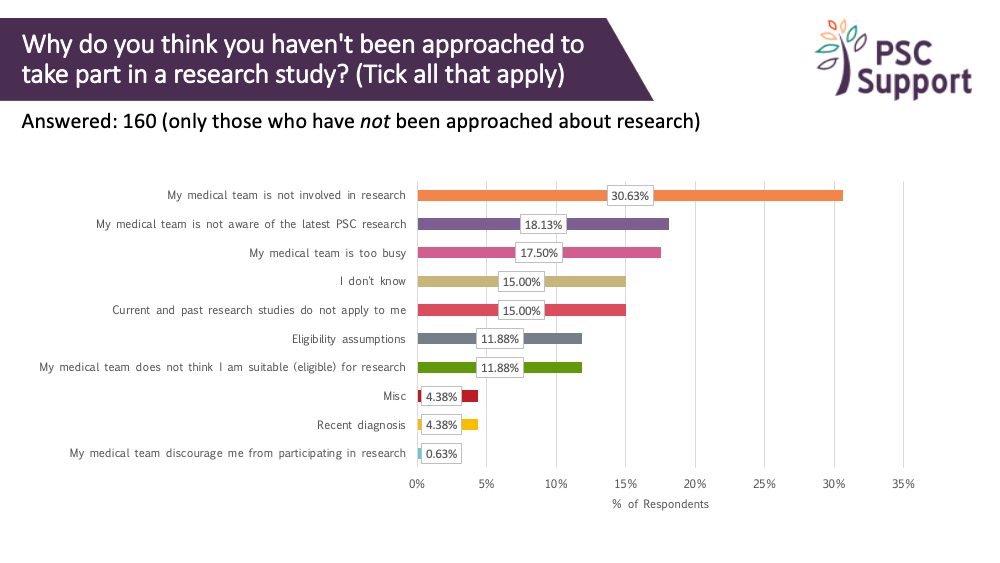
Of those who had not been approached to take part in research, nearly one in three (30.63%) thought this was because their medical team was not involved in research. Nearly one in five (18.13%) thought that their medical team was not aware of the research and/or was too busy (17.5%).
Of concern some assumed that they were not eligible.
Recommendation 3
All patients who are eligible to take part in clinical trials should be proactively contacted with information about the trial. Many assume that they will be automatically contacted and if they are not, incorrectly believe that they do not meet the eligibility criteria. Arrangements must be made to quickly and reliably respond to enquiries from patients who proactively reach out to study teams and hospitals about trials.
Recommendation 4
Patients place great value on the opinion of their consultants. Efforts should be made to enable the healthcare professionals who care for PSC patients (particularly those not involved in research) to make appropriate referrals of patients for trials.
Supporting insights
The logistics of taking part
Attending a different hospital for research
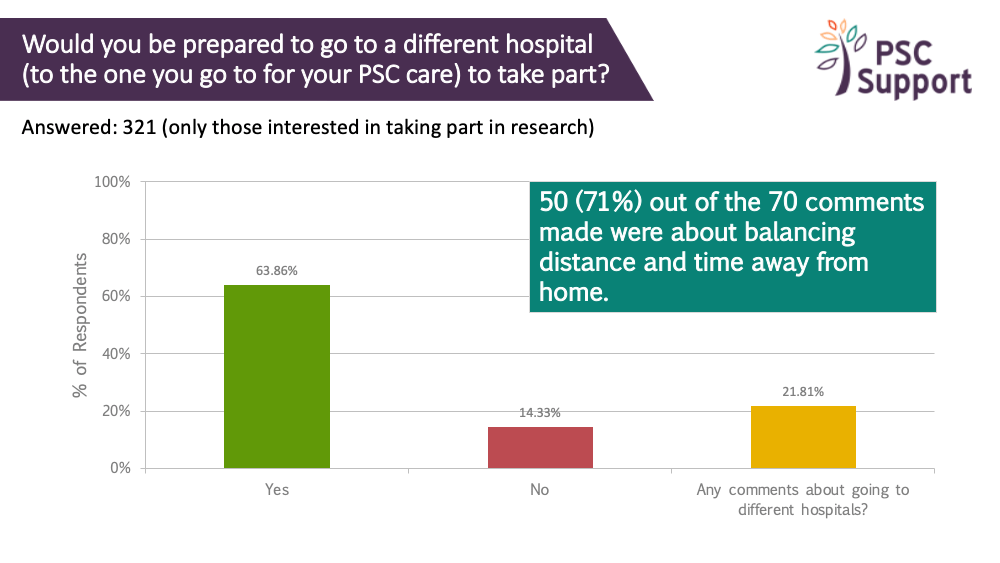
Two out of three people (64%) who were interested in taking part in research were prepared to change hospitals to take part in a research study. People took a pragmatic approach and most of the 70 comments (71%) expressed the need to balance distance and time away from home.
People were also keen to have the support of their own PSC doctor.
Recommendation 5
Patients are prepared to attend different hospitals to take part in research studies. Transfer to different hospitals for clinical trials should be co-ordinated with each patient's regular PSC doctor and fully supported by the whole team.
Two out of three people (64%) who were interested in taking part in research were prepared to change hospitals to take part in a research study.
Patients said:
"Yes as long as I can afford and manage travel."
"Yes if it's a reasonable distance and does not interfere with work"
"As long as my hepatologist approved."
"Would still want my current hospital informed but would be concerned that my psc care is not interrupted and hospitals communicate so I don't have to be the link person doing updating on 2 sides"
Travelling
We asked how far people would travel to take part in research, if travel costs were covered.
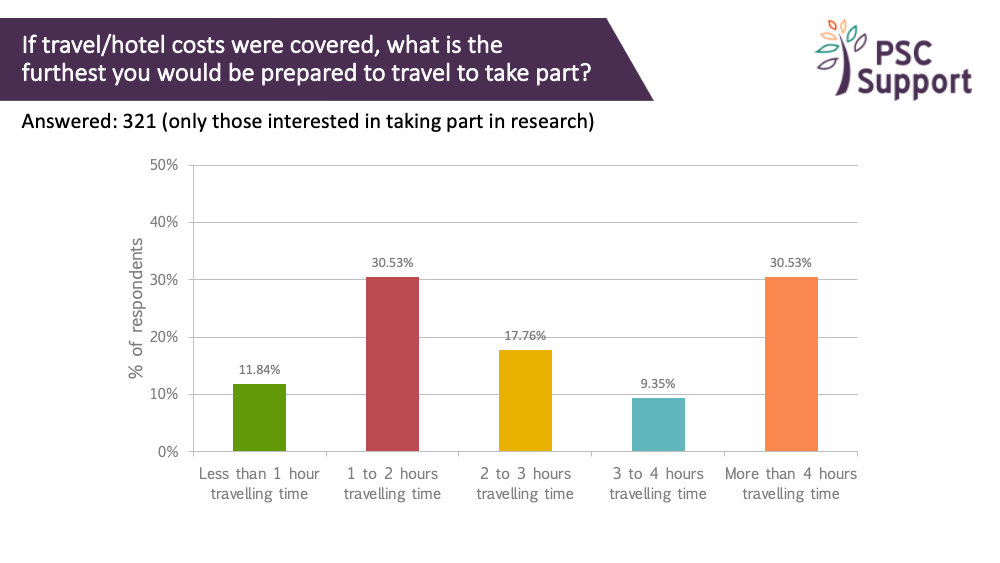
Four out of five (81%) were prepared to travel for at least an hour to take part in research, if travel costs were covered (and a third for at least four hours).
Four out of five (81%) were prepared to travel for at least an hour to take part in research, if travel costs were covered.
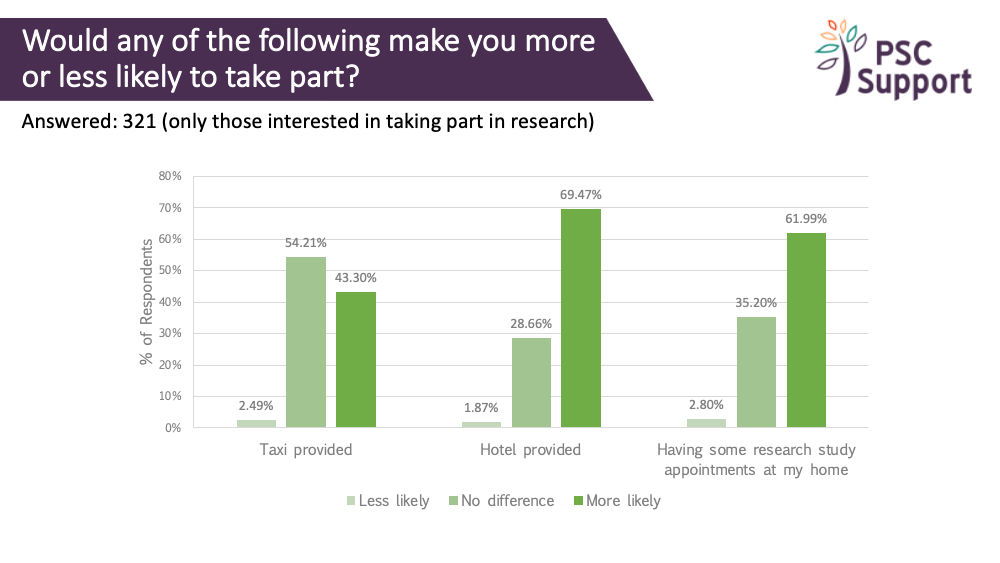
69% indicated that they would be more likely to take part if a hotel was provided (and for a quarter, 29%, it made no difference).
Hotels
69% indicated that they would be more likely to take part if a hotel was provided (and for a quarter, 29%, it made not difference).
Patients said:
“As long as not too expensive for whoever pays.”
“Due to fatigue a hotel would help if I had to drive to an appointment.”
“Only if it was considerably far away.”
62% said having some study appointments at home would make them more likely to take part, and it made no difference for a third (36%).
Home visits
62% said having some study appointments at home would make them more likely to take part, and it made not difference for a third (36%).
Patients said:
“Would be great, but not a deal breaker.”
“Or in a local convenient place.”
“…research by telephone conference or call works well, otherwise cost is very prohibitive if required to travel...”
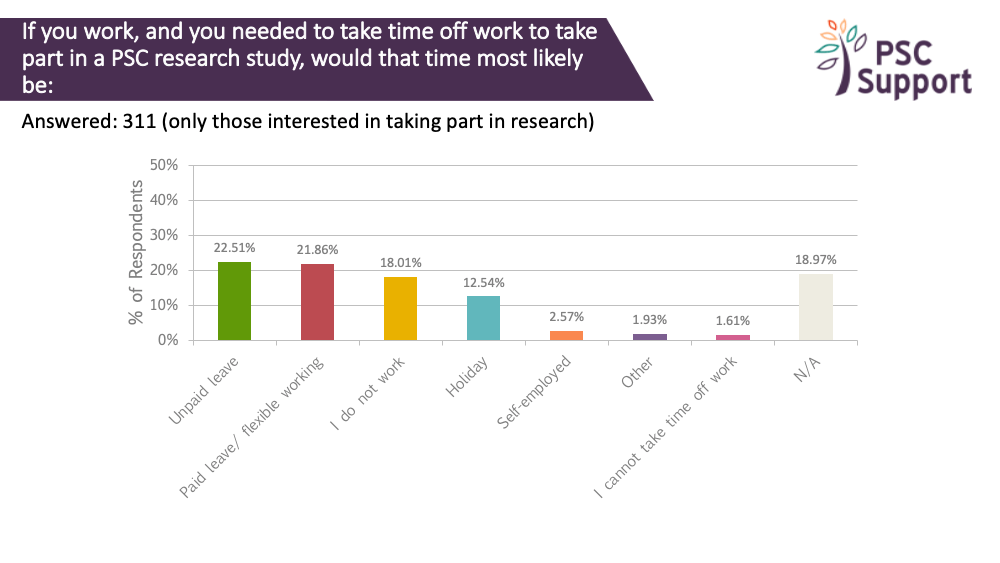
Time off work
We asked how people would be likely to take time off work to take part in PSC research. 311 people answered this question. Only 19% said this question wasn't applicable to them, and 18% indicated that they didn't work.
23% indicated that they would use unpaid leave to go on a research study. Another 22% said they would take paid leave or use flexible working to take part. Another 12% said they would use holiday.
One patient said:
“Would look to minimise any effect on work and time taken off overall, this would be a priority in making decision to take part.”
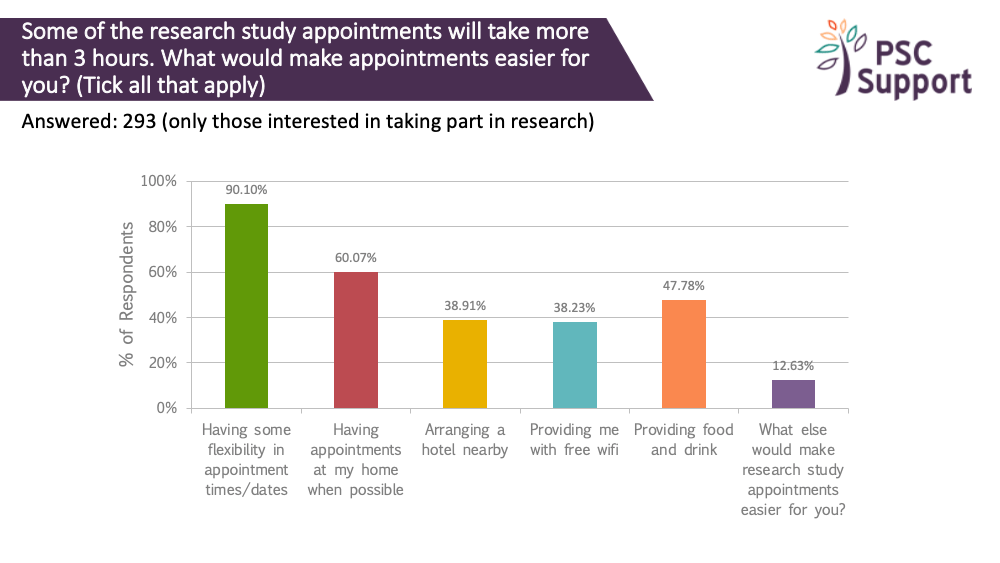
Making study visits easier
Nine out of ten (90%) respondents said having some flexibility in appointment times and dates would make things easier. Two thirds (60%) said having them at home would make things easier.
Recommendation 6
We recommend that study visits are planned so that they are at convenient times for participants, and close to home when possible. Not every study visit needs to be in a research centre. Study team members could visit participants' homes or places of work to remove a huge barrier to participation, or arrange for local testing. Where medical procedures and tests are not required, the research team should seriously consider the use of video conferencing. In light of the COVID-19 pandemic, this recommendation is more important than ever.
Supporting insights
The costs of trial participation to patients
Out of pocket expenses
Travel costs
Travel costs should be reimbursed to participants. As seen above, participants are prepared to travel long distances if travel costs are covered.
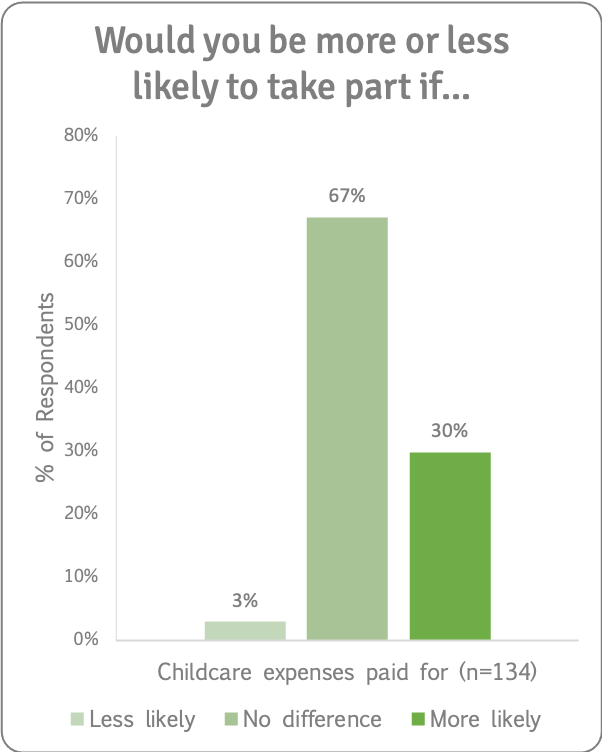
Childcare
We asked if having childcare expenses paid for would make people more or less likely to take part in a research study. Of those for whom this was applicable, a third said this would make them more likely to take part (and two thirds said it would make no difference).
One patient said:
“My mum helps a lot but does work and I've got to be realistic I can't afford more childminding costs than I already have.”
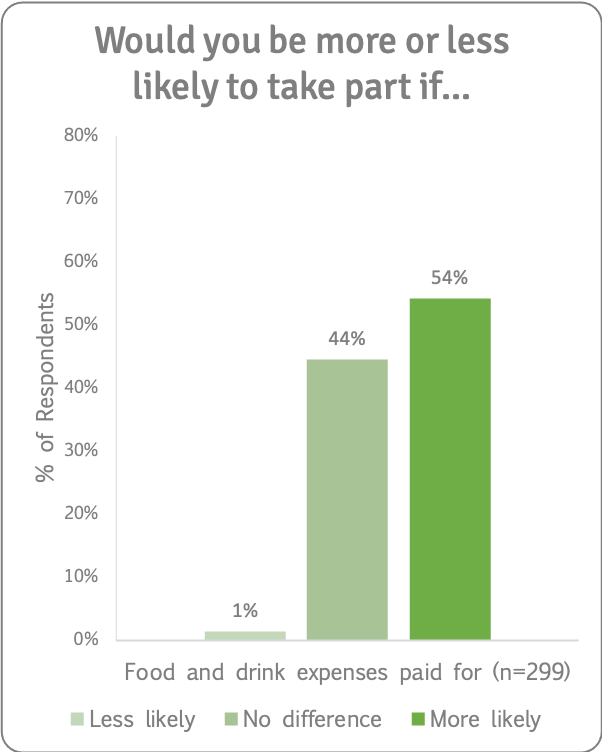
Food and Drink
We asked if having food and drink expenses paid for would make people more or less likely to take part in a research study. 311 people answered this question. 12 people said this wasn't applicable to them. Of those for whom this was applicable, half (54%) said that they would be more likely to take part in a research study if food and drink expenses were paid for and half (44%) said it would make no difference.
One patient said:
“A small budget for this expense would be a positive but not necessary.”
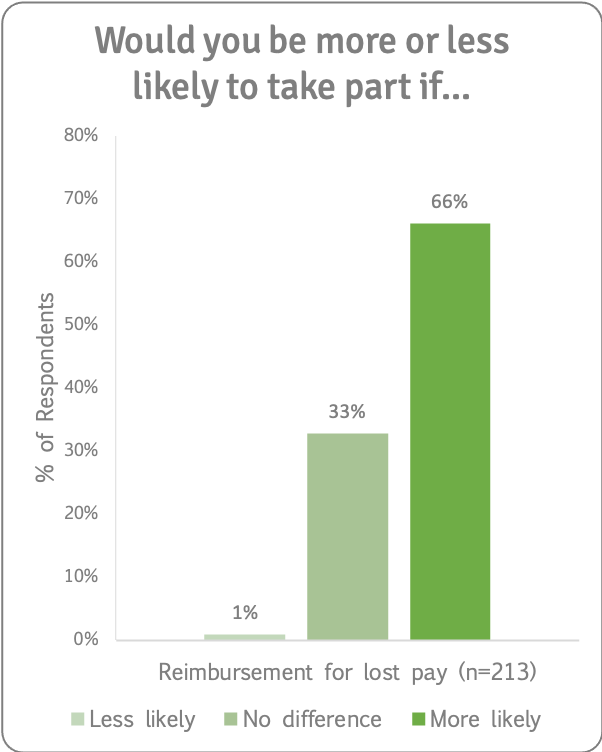
Lost pay
We asked if being reimbursed for lost pay would make people more or less likely to take part in a research study. Of those for whom this was applicable, two thirds (66%) said that they would be more likely to take part in a research study if lost pay was reimbursed. One thirds (33%) said it would make no difference.
One patient said:
“If it was the right study that I felt would really help people then if I had the funds to cover not working at the time then I would be willing to do it.”
Other costs
We asked what other costs people would incur if they tool part in a research trial. They could be categorised into five broad themes:
- The cost of partner accompanying the person with PSC.
- The costs of arranging care for other dependents including pets.
- The cost of using up annual leave.
- Costs associated with employment: suggestion that a formal letter explaining the importance of the research to employers be provided.
- The human cost: time and effort and impact on fatigue.
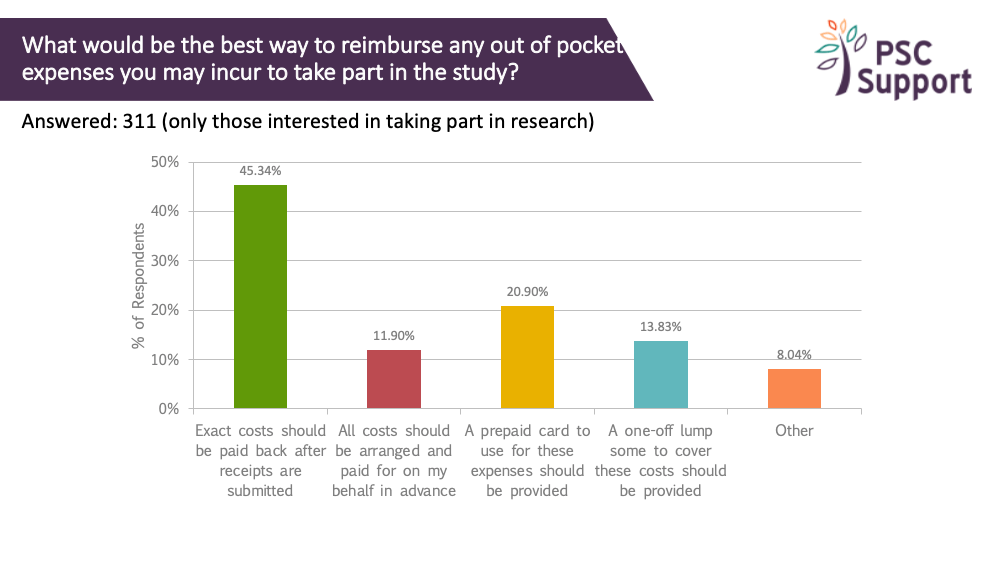
Reimbursement
We asked about the best way to reimburse and out of pocket expenses.
Half (45%) said that exact costs should be paid back after submitting expenses. Others preferred that the costs be prepaid. Only 14% thought that a one-off lump sum was the best way to reimburse out of pocket expenses.
Recommendation 7
Study sponsors should seriously consider the cost to participants when they take part in clinical trials. People with PSC are typically trying to hold down full time jobs and the cost of taking part in clinical trials goes far beyond out of pocket expenses. The cost of taking time off work should be reimbursed to participants if they work, as well as reasonable out of pocket travel and sustenance expenses.
Recommendation 8
It is important that the system used to reimburse expenses and lost pay is robust, fast and simple.
Patients said:
“I don’t really mind as long at it was easily accessible and was a smooth process...”
“If there weren’t too many appointments I would feel bad to claim expenses so I would prefer to submit receipts so I can decide what I’d like to be reimbursed for.”
Supporting insights
Study design
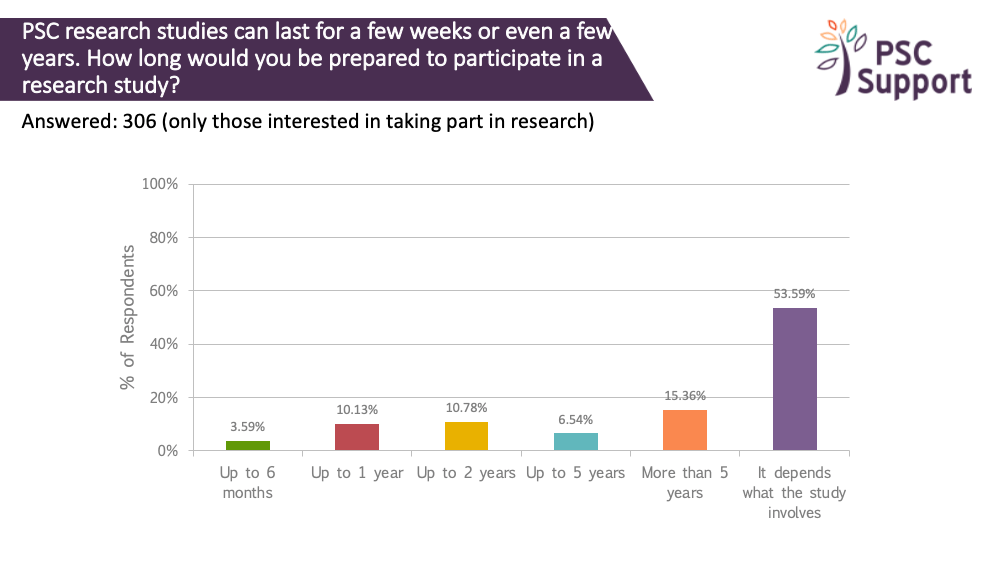
Length of a study
PSC research studies can last for a few weeks or even a few years. We asked how long people (who were interested in taking part in research) were prepared to participate in a study.
A third were prepared to take part in studies that lasted up to two years (and some of those were prepared to take part for over five years).
However, half (54%) indicated that it would depend on what the study involves.
Recommendation 9
Longer PSC clinical trials are feasible as long as the reasons for the length are clearly explained to potential participants.
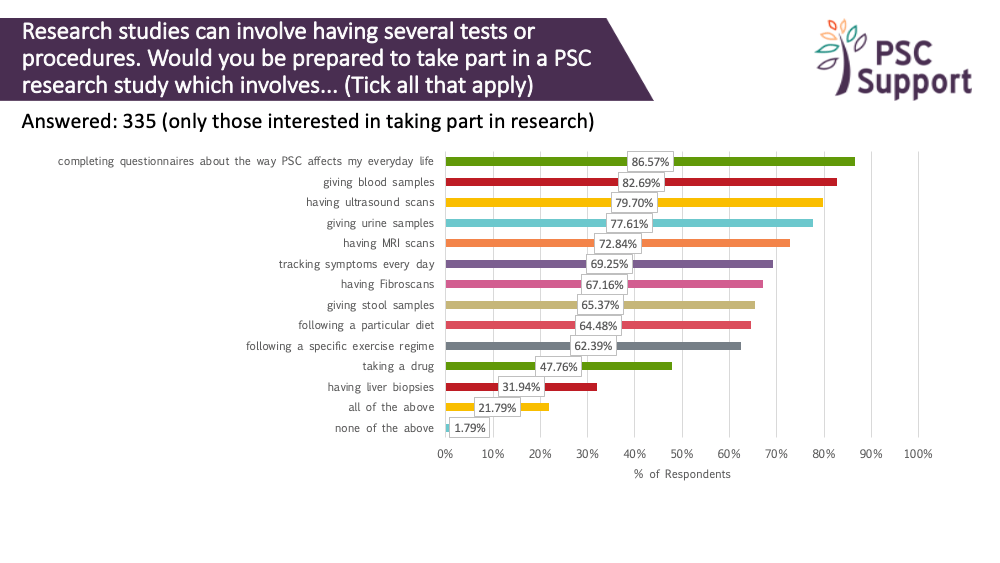
Tests and procedures
Research studies can involve having several tests or procedures. We asked if respondents would be prepared to take part in a PSC research study which involved various procedures and tests. Most people were prepared to take questionnaires (87%) and track daily symptoms (69%), have blood tests (83%), ultrasound scans (80%), MRI scans (73%) and Fibroscans (67%), give stool (65%) and urine (78%) samples, and even follow specific diets (64%) and exercise regimes (62%). Contrary to our previous survey results, only 32% of respondents were prepared to have a liver biopsy for research. We believe that context plays a part here, and sharing the reasons for using biopsies and their importance in PSC trials is critical to mitigate this barrier to participation.
Recommendation 10
People with PSC show willingness to undergo tests and procedures, and to complete questionnaires in clinical trials. We recommend that the reasons for the use of biopsies and other invasive procedures are clearly explained and justified for each clinical trial.
Placebo and active treatment drugs
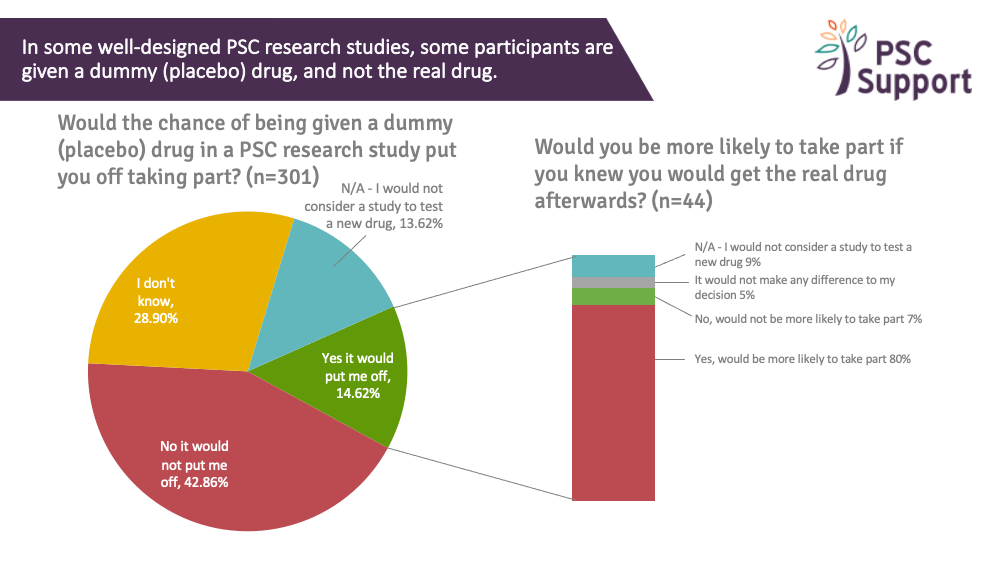
Of those interested in taking part in research, 15% said the chance of being given a placebo (dummy) drug in a PSC research trial would put them off taking part but 43% said it would not put them off and a third (29%) said they didn't know if it would put them off.
Half (50%) said yes, they would be more likely to take part if they knew they would get the real drug afterwards, and a third (31%) said it would make no difference.
Interestingly, of the 44 people (15%) who said they would be put off by the chance of having a placebo drug in a trial, 80% said they would be more likely to take part if they knew they'd have the option to take the real drug afterwards:
Recommendation 11
Participants should be given the opportunity to take the treatment drug after the trial if they are in a placebo group, should the active drug prove to be effective. Thought should be given to innovative trial design that allows all participants the chance to take the study drug at some point.
Reducing or stopping existing medications
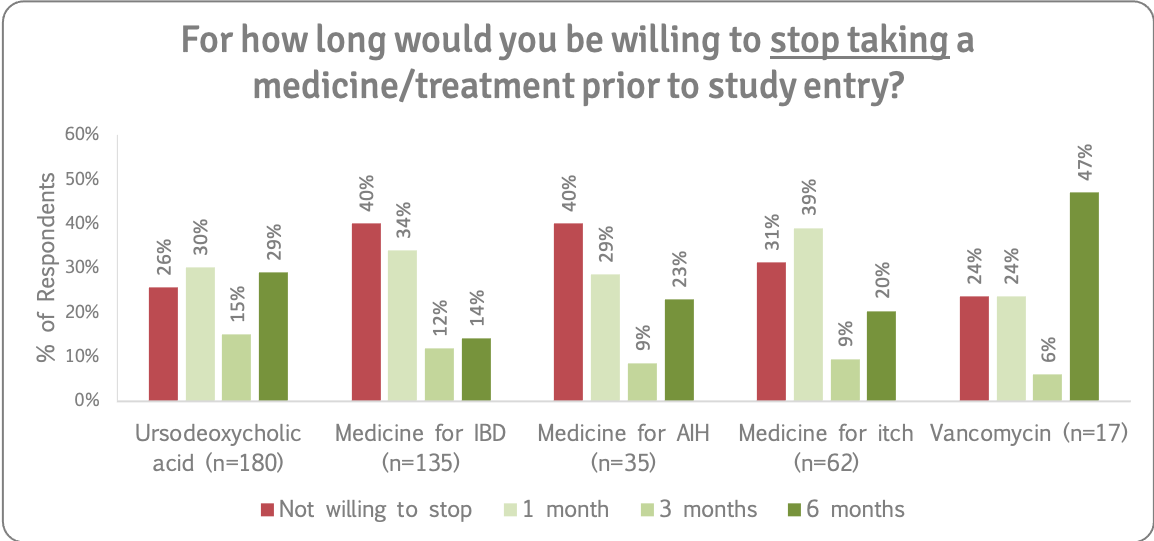
In some well-designed PSC research studies, participants must reduce or stop taking some of their current medicines for a minimum period of time before the study starts.
It is important to note that patients should never reduce the dose or stop any prescribed medication without prior recommendation or guidance from their consultant/ research study team.
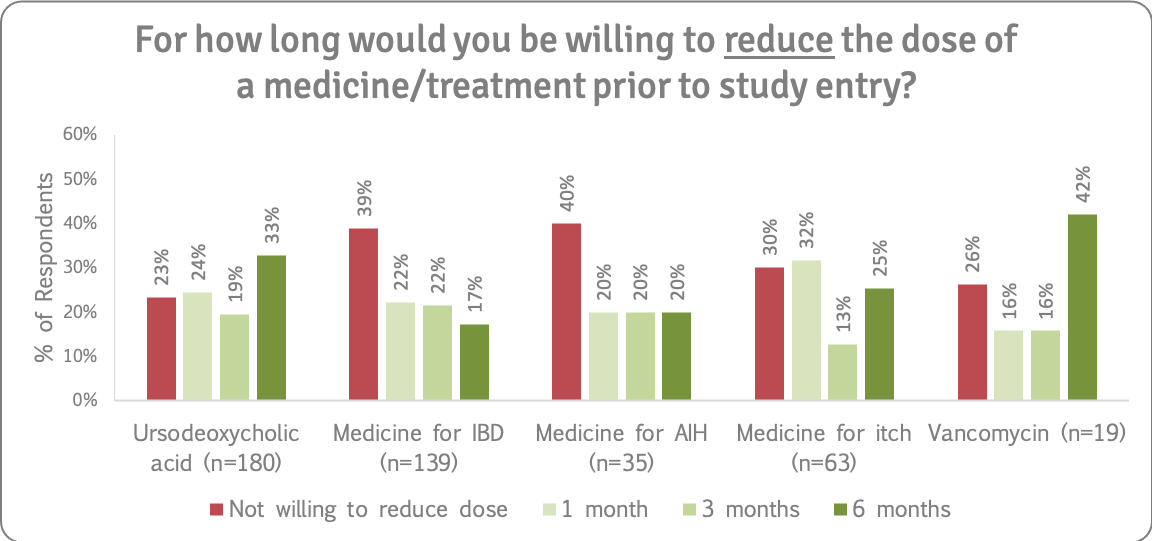
We asked how far ahead people would willing to stop taking or reduce the dose of AIH, IBD and itch drugs, as well as UDCA and vancomycin, prior to study entry. Refer to the supporting insights for more details.
Patients said:
“Reducing IBD meds is scary as I wouldn't want to upset that whilst it's behaving.”
“I would be concerned about stopping anything which would affect disease progression so I would need to be shown that additional monitoring would be provided to check that this isn't the case.”
“I can reduce or stop it, but would need alternatives if the itch becomes persistent.”
“Would need to discuss consequences with medics.”
Recommendation 12
People with PSC will not blindly reduce or stop taking existing medications and prefer to make informed decisions. There is a strong willingness to reduce/stop medications for research with support of their medical/ clinical trial team. Given the fact that people with PSC live with other conditions, eligibility criteria should include patients who take prescribed medications if they are on a stable dose and not experiencing a flare-up of any condition, or at the very least, trials should include these groups of people in longer term phase IV studies/open label extensions evaluating the treatment effectiveness, in real world conditions.
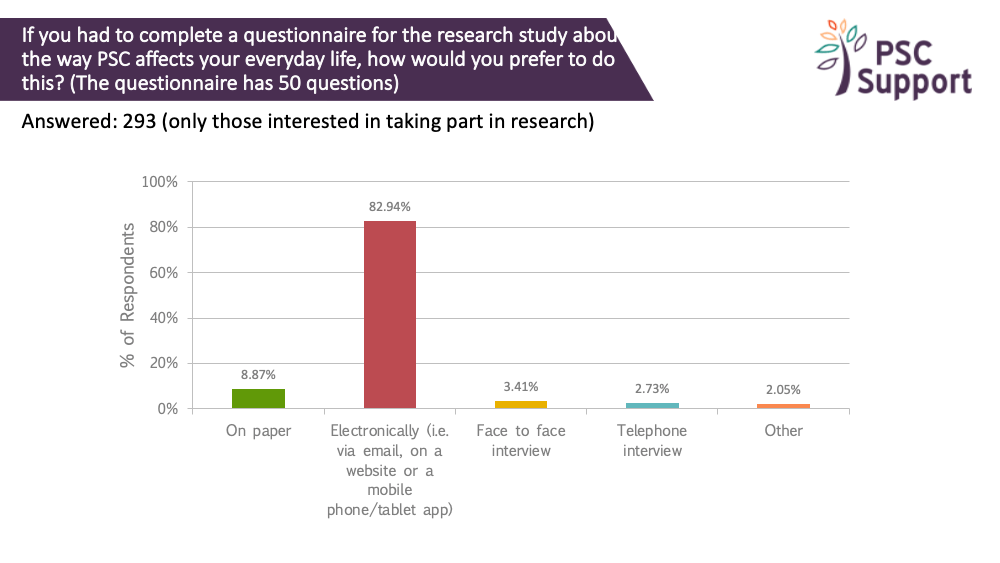
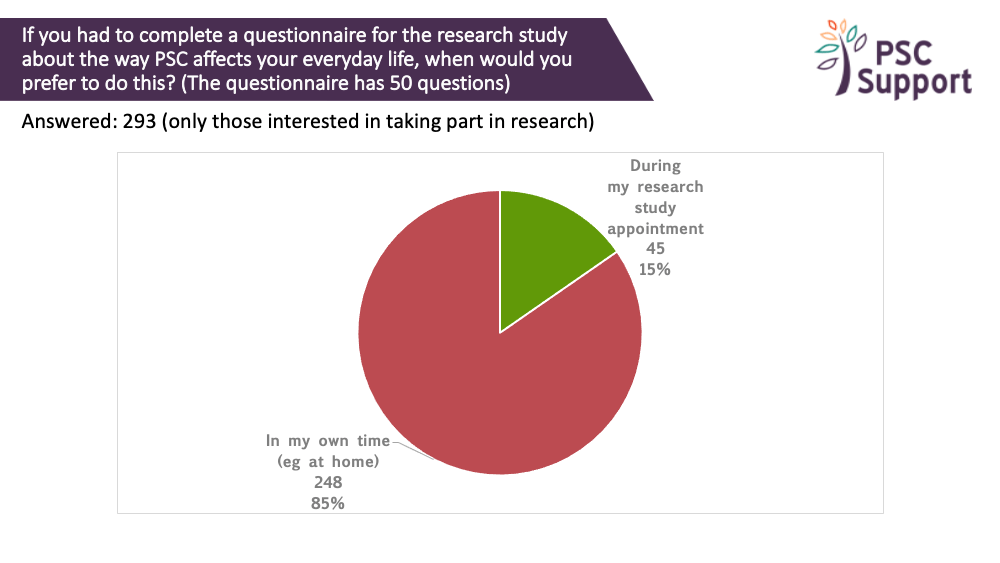
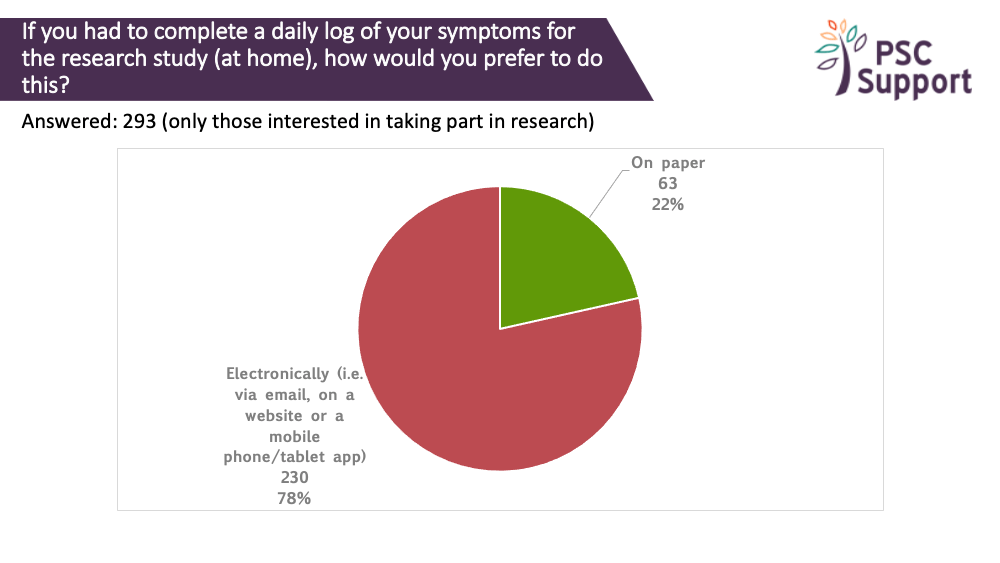
Paper or electronic questionnaires?
Many studies incorporate questionnaires about the way PSC affects everyday life. The questionnaires can sometimes have 50 or more questions.
Most (83%) of those interested in taking part in research said they would prefer to complete such questionnaires electronically, such as via email, on a website or using mobile/tablet app, and do so at home (85%).
83% of those interested in taking part in research said they would prefer to complete such questionnaires electronically, such as via email, on a website or using mobile/tablet app. 9% said they preferred to complete the questions on paper.
85% of those interested in taking part in research preferred to complete them in their own time (e.g. at home). 15% said they would prefer to complete them during their study appointment.
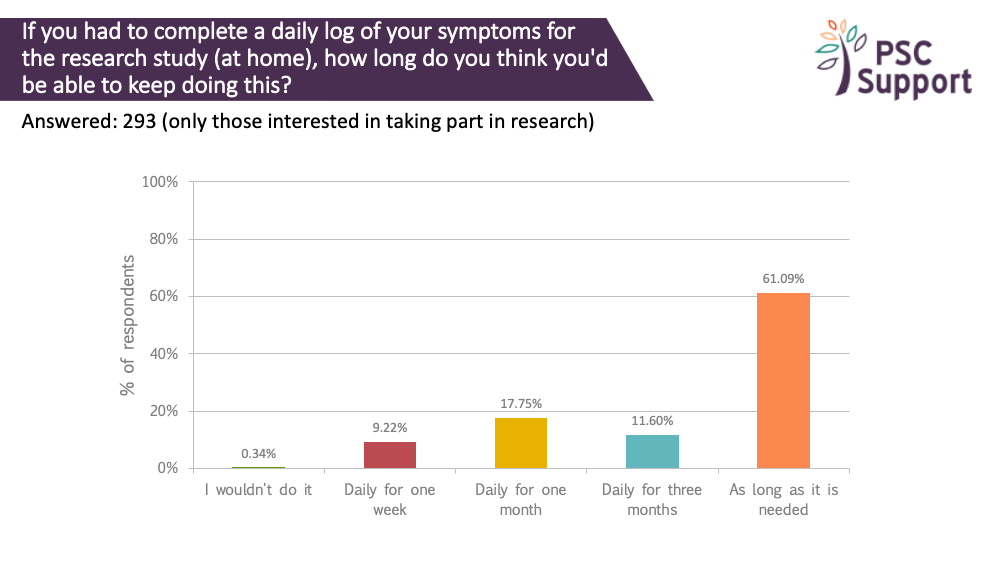
There was a strong willingness to track daily symptoms. 99.66% were prepared to log symptoms daily for a week, 90% said they would log them daily for a month, 73% daily for three months and 61% were prepared to log daily symptoms for as long as was needed. Again, most people (78%) preferred to do this electronically.
Recommendation 13
Quality of life questionnaires presented to participants to complete during study visits (often when they have been fasting) will not elicit a true reflection of how the participants are feeling and functioning. We strongly recommend offering the option to complete quality of life and other questionnaires electronically, at home.
Contact between study visits
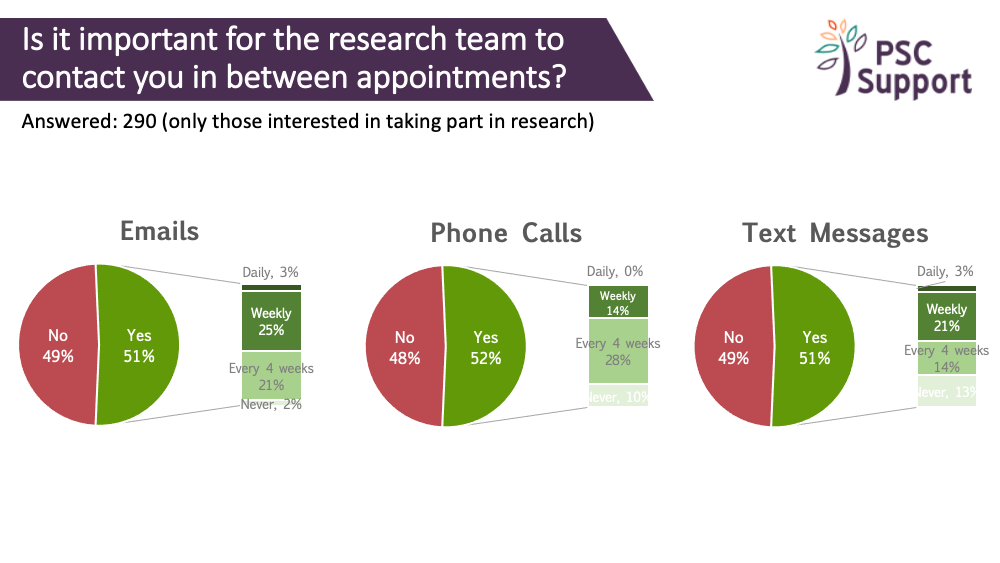
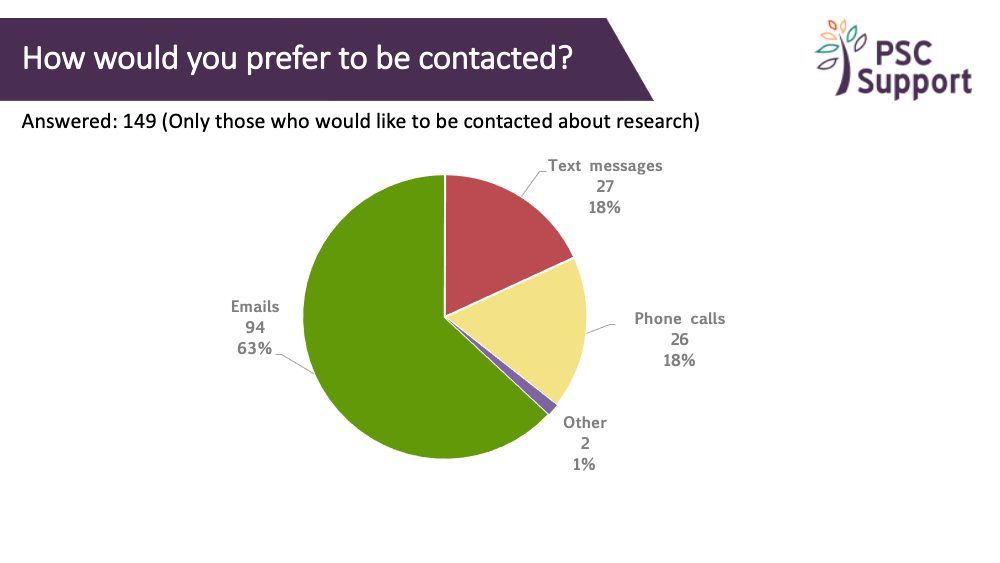
We're often asked about frequency of contact between study visits. Respondents were split as to whether contact between appointments was important or not.
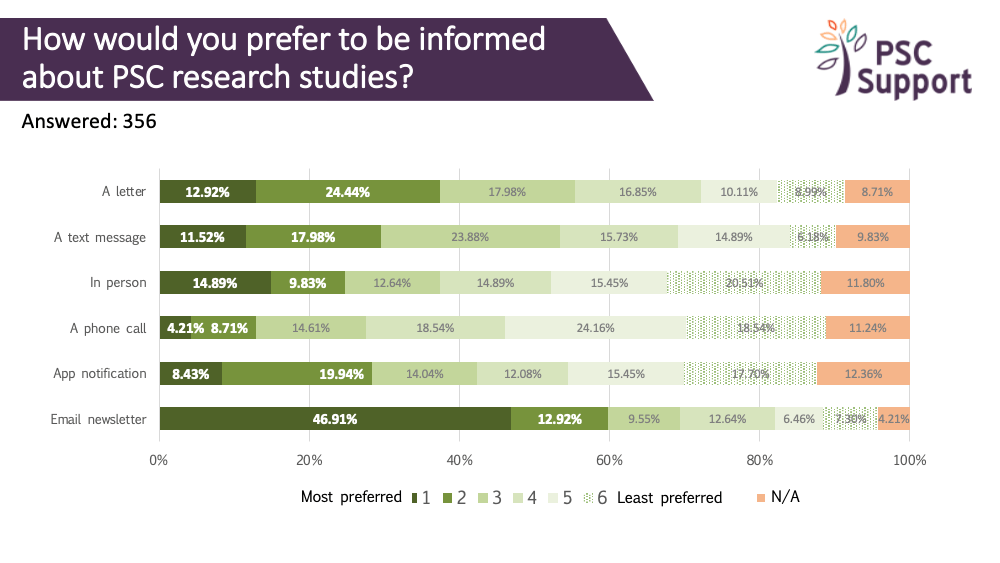
Email contact was preferred weekly or monthly (4 weeks), 4-weekly phone calls were preferred (no one wanted a daily phone call!) and weekly text messages were preferred.
Recommendation 14
Individual preferences on method and frequency of contact from the trial team between study visits should be obtained at the beginning of every clinical trial.
The charts show the proportion of respondents who found contact between study visits important/not important, and for those who said it was important, what frequency of contact they thought was appropriate, and how.
Clinical trial results
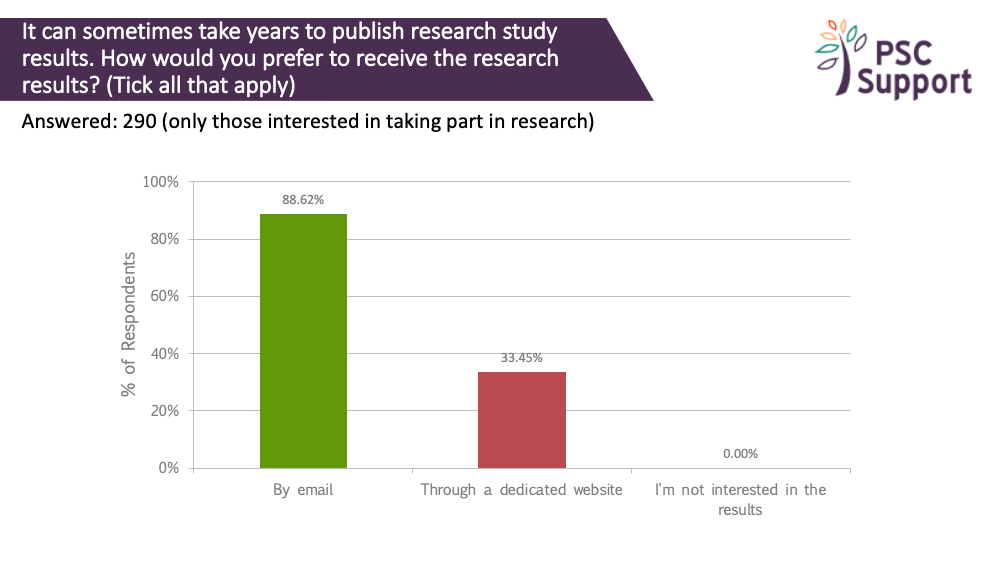
It can sometimes take years to publish research study results. Everyone wanted to know the study results. Most people (89%) said they would like to be emailed with study results, and 33% said they would prefer to find out via a dedicated website.
Recommendation 15
All participants should be invited to sign up to a mailing list specifically for sharing future study results. Like any mailing list, they should be able to have the option to unsubscribe and manage their own contact preferences.
Suporting insights
PSC Support Patient Insights Report (Part 2 - PSC Clinical Trials) was written by PSC Support and published 17 August 2020.
Date updated: 19 August 2020