PSC Support represents patients in PSC research and medicine development
PSC Support is leading the way in driving and supporting efforts to find a cure for PSC

Thank you
Thank you to everyone who takes the time to complete PSC Support surveys. Your views are making a difference and have helped us demonstrate the unmet needs of patients and improve clinical trial designs, each time taking us a step closer to an effective treatment. We only ever ask essential questions, and always make your answers count.
As well as funding research, PSC Support represents people with PSC nationally and internationally in our work to speed up the development of an effective treatment.
These are just some of snapshots of the extensive work we are doing to support the research community to develop an effective treatment for PSC. Martine Walmsley has led PSC patient organisations in international consultation responses, research prioritisation, clinical trial design and quality of life improvement. We regularly work with PSC researchers to develop research applications and study protocols, prepare patient-facing information for clinical trials and sit on trial steering committees to ensure the best PSC and liver research can go ahead. PSC patients need to live longer, and better, and we're committed to making that happen.
Making clinical trials more accessible
Guide to Clinical Trials
The ONLY way to find a treatment, cure, or achieve better control of symptoms for PSC is through well-designed clinical trials. We believe that people with PSC should be fully informed about how clinical trials work, what’s involved and why they’re so important.
We've taken questions about PSC research and answered them in our new Clinical Trials Guide. It contains in-depth information about taking part in clinical trials, especially for patients who are seen in a hospital that isn't running a trial, what's usually involved and why, questions to ask before deciding and more.
Patient Insights Report
PSC is a rare disease and every clinical trial risks being unable to recruit the necessary numbers to demonstrate efficacy of the treatment drug, despite the positive attitudes from patients about taking part. If trials don't recruit enough patients, we will never get a cure.
We have seen a demand from patients for clinical trials, but there are a number of barriers preventing or putting off otherwise willing and eligible participants from taking part.
The PSC Support Patient Insights report identifies motivations for and barriers to taking part in research and makes recommendations about how researchers and pharmaceutical companies can change trial design to maximise recruitment.
Raising the international profile of our rare disease
International Liver Congress 2020
PSC Support is proud to be speaking at the Opening Ceremony at the first digital International Liver Congress (ILC), a forum for the most ground-breaking hepatology research in the world. In 2019, the ILC brought together 10,000 delegates from around the world:
International Liver Congress 2019
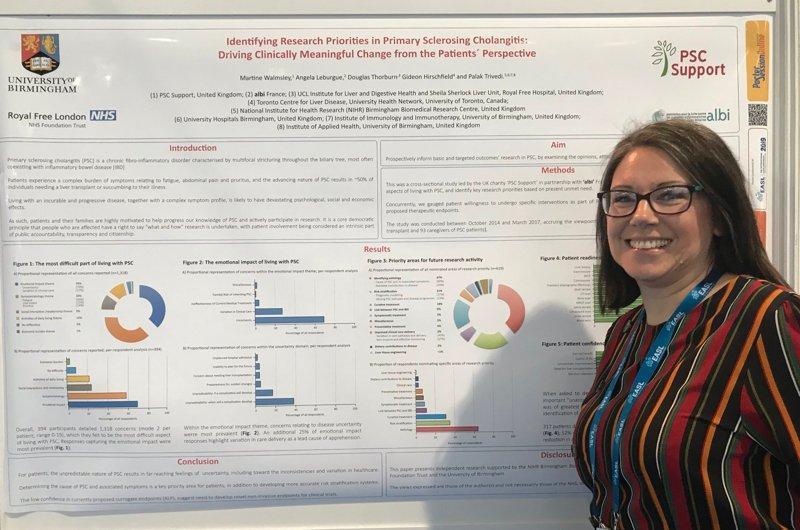
In April 2019, we presented a poster with ALBI France at the EASL International Liver Congress demonstrating the results of our patient surveys140 attended by thousands of scientists, healthcare professionals and researchers from all over the world. Many thanks to Professor Hirschfield, Dr Trivedi and Professor Thorburn for their support.
Presenting the PSC patient perspective to medicine regulators
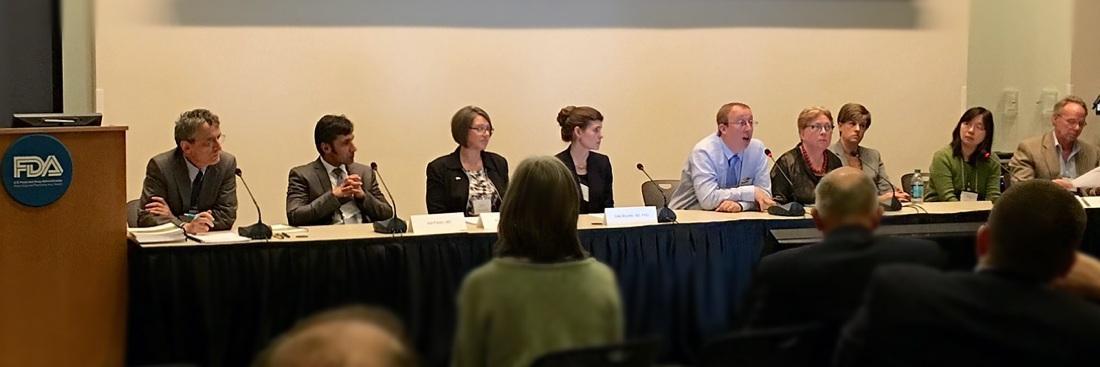
2016 - The US Food and Drugs Administration (FDA)
We evidence the views we provide. Two PSC Support surveys capturing more than 1,300 respondent views of people affected by PSC have been used as a basis for new research, cited in landmark scientific papers on PSC, and presented to the FDA in March 2016 when PSC Support was invited to represent all PSC patients at an international conference hosted by the American Association for the Study of Liver Diseases (AASLD) and the US Food and Drug Administration (FDA). Our report is called ‘Clinical need in PSC and clinically meaningful change: What is important to patients?’35. The PSC Forum was created as a result of this important conference to bring together all stakeholders in PSC medicine development to speed up our search for an effective treatment.
PSC Support Presentation Slides
2018 - The European Medicines Agency (EMA)
In December 2018, PSC Support was invited to present the patient perspective on PSC medicine development with PSC Patients Europe at another international meeting: the 2018 European Medicines Agency multi-stakeholder meeting on PSC, PBC and NASH research.
Key Facts and Figures
PSC Support Research and Treatment Surveys (interim findings from 2016)
Take Home Message
- PSC patients are interested and eager to participate in research studies.
- Patient-centred research design will help recruitment and retention of participants.
- Patient Reported Outcome Measures should be incorporated into trial design where relevant.